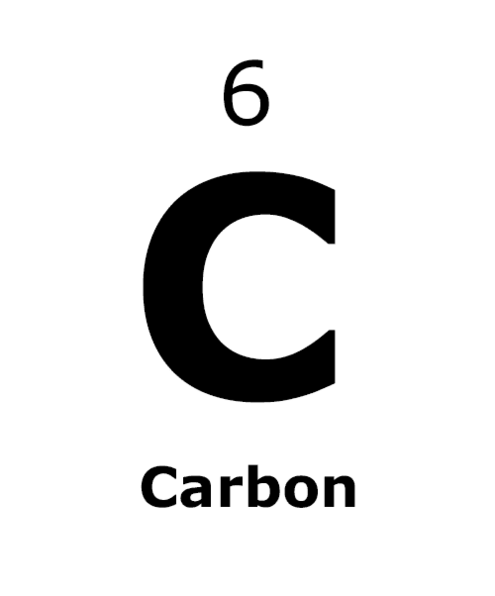
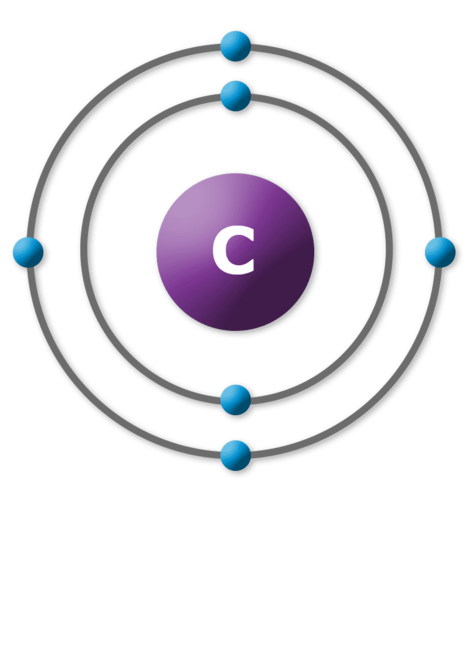
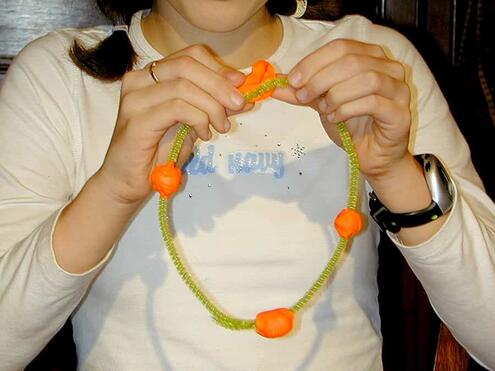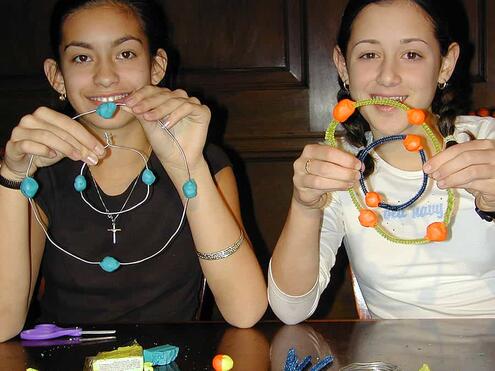Take a look around you. What do you see? Your desk, your hand, a tree out the window. Everything you can name is matter.
What is Matter?
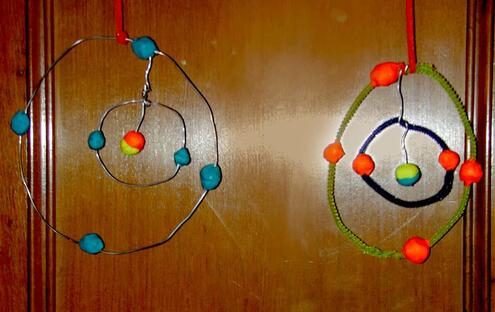
Make a mobile of a carbon atom!


-
Periodic Table
(Click here to get the pdf.) - Scissors
- Wire and pipe cleaners
- Clay (3 different colors)
- Thin string, thread, or nylon thread (optional)

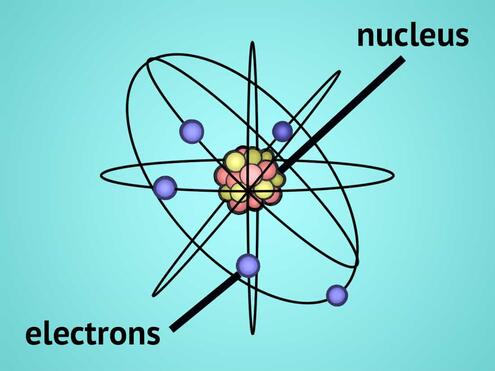
Atoms are made of even smaller parts: a central nucleus made of protons and neutrons. Electrons orbit in spheres around the atom.
Try This!
Did You Know?
Image Credits:
All Images: courtesy of AMNH




 Biodiversity
Biodiversity
 Brain
Brain
 Genetics
Genetics
 Marine BiOLogy
Marine BiOLogy
 MicrobiOLogy
MicrobiOLogy
 PaleontOLogy
PaleontOLogy
 ZoOLogy
ZoOLogy
 AnthropOLogy
AnthropOLogy
 ArchaeOLogy
ArchaeOLogy
 Astronomy
Astronomy
 Climate Change
Climate Change
 Earth
Earth
 Physics
Physics
 Water
Water