Making Rocks

Hi, I’m Jim Webster . I’m an Earth scientist at the American Museum of Natural History and I study volcanoes.
Some volcanoes slowly ooze magma like a thick stew that overflows on a hot stove. The ones I study explode with immense power.
Like most scientists who study volcanoes, I visit them to get a closer look and collect rock samples. But my real research happens in the lab. I'm interested in where all the action starts—way down below the volcano in the magma chamber, where gas-rich magma collects. As you can imagine, it's way too deep and hot to actually go there. One way I can get a close-up look is by recreating the conditions of a magma chamber in my lab. If I can do this, I can understand what it is about magma that makes some volcanoes so explosive.
Introduction
Before we get started in the lab, there are a few things to know about volcanoes.
Magma is hot, melted rock found inside the Earth . It collects deep under a volcano in an area called a magma chamber. Over time, magma and gases like water vapor and carbon dioxide build up in the magma chamber. When the pressure becomes too great, a volcano will erupt.

You can think of a magma chamber as a bottle of soda. The soda is like the magma and the fizz represents all the gases in the magma. When the cap is on, the fizz is under a lot of pressure, but it is dissolved in the magma.

When you crack open the soda bottle, the pressure is released, or suddenly lowered. As the gases try to come out of the soda, bubbles are formed. As soon as bubbles form, they want to escape the liquid. This causes an explosion.
In the same way, when a crack opens up above the magma chamber, the magma bursts out in an eruption. When a volcano is explosive, we know there were lots of gases dissolved in the magma.
I want to find out how various gases behave in magma at different temperatures and pressures. When do the gases dissolve in magma? How are the gases released? When do they cause an explosive eruption?
My goal is to understand, and even predict, explosive volcanic eruptions. This could protect the 500 million people across the planet who live near active volcanoes!
Step 1: Visit Volcanoes and Collect Rocks
I may do most of my work in the lab, but my research starts in the field, at actual volcanoes.
I study two volcanoes: Mount Vesuvius in Italy and Mount St. Augustine in Alaska. Both these volcanoes erupted explosively many times. They're both found above subduction zones, places where the edge of one tectonic plate is sinking underneath another.

The explosion of Mount Vesuvius in A.D. 79 buried the cities of Pompeii and Herculaneum with mud and ash.

Mount St. Augustine is part of the Ring of Fire , a chain of volcanoes circling the Pacific Ocean. It has erupted twice since 1969.

I hope to discover what makes these types of volcanoes explosive. I start by collecting pumice, a light rock filled with holes like a sponge. Pumice forms from explosive eruptions. The lava flows during these eruptions are foamy from all the gas. The frothy liquid cools very quickly. When it hardens, the bubbles in the lava become holes in the solid rock. In fact, there are so many holes that pieces of pumice actually float on water!
Step 2: Examine the Rocks in the Lab
Now I return to the lab to study the pumice. This is where my research really begins!
These rocks formed over thousands of years under extreme temperatures and pressures. My goal is to figure out exactly how they formed. To do this, I'm going to crush a piece of pumice and try to re-create it in the lab. I have to learn as much as I can about the rock before I begin.
I'm most interested in two parts of the pumice: volcanic glass and melt inclusions.

Volcanic glass is magma that cooled quickly after it erupted. It has the same composition as the original magma, minus gases that escaped.
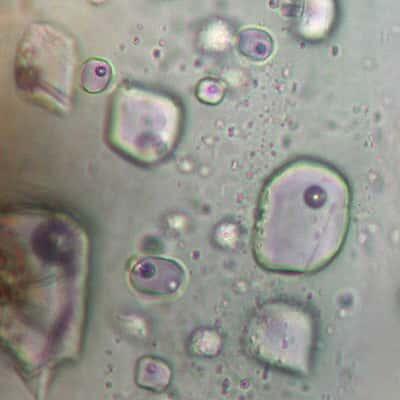
Down in the magma chamber, sometimes drops of melt, or magma, get trapped inside minerals as they grow. When the magma erupts and cools, these melt inclusions remain intact and preserve droplets of the original magma, including the gases.

My scientific assistant Christine Tappen does most of the work to get the samples ready. She also helps to analyze them.
To begin, we prepare the samples.
To get a closer look, we cut a rock into thin slices or crush it and separate the grains. Then we mount the samples on glass slides.
Then, we examine them.
We're looking for rock samples with minerals that contain melt inclusions. These can only be seen with a powerful microscope because they are smaller than a grain of sand. We also use more powerful instruments to measure the amount of and types of gases in the samples.
Now I know a lot about the pumice. I'm ready to begin my experiments.
Step 3: Make a Mini-Magma Chamber
What was it like deep in the magma chamber when the pumice formed? What was the temperature? What was the pressure?
We experiment with different conditions until we have re-created the same rock. Then, we will know more about what it was like deep inside the magma chamber, before the volcano erupted.
FIRST, we prepare a mini-magma chamber

We combine all of the ingredients needed to make our own rock.
- We crush some of the pumice we collected into a fine powder.
- This goes into a tiny gold capsule, with some gas we added.
- Then, we seal it tight so the gas does not escape.
SECOND, we turn it into magma...and make a rock!

We put our capsule, or mini-magma chamber, under some pretty extreme conditions.
- We heat the sample to about 800 degrees C (1500 degrees F.) That's about three times hotter than your oven gets.
- We set the pressure to about 2000 bars. This is equal to the pressure exerted by the Earth about 4 1/2 miles deep.
- After the machine runs for several days, we shut it off so the magma cools quickly and hardens into glass.
THIRD, we analyze our new rock.
If it has the same amount of gas as the original rock, we know we chose the right temperature and pressure. If not, we try again.
It can take many experiments to discover the same conditions as the original magma chamber. But, every time that we do it, we are learning more about what makes volcanoes explode.
Image Credits:
erupting volcano and soda bottle illustrations, courtesy of AMNH / Eric Hamilton; Pompei and Mt. Vesuvius, courtesy of Qfl247 via wikimedia commons (CC BY-SA 3.0); all other photos, courtesy of AMNH.




 Biodiversity
Biodiversity
 Brain
Brain
 Genetics
Genetics
 Marine BiOLogy
Marine BiOLogy
 MicrobiOLogy
MicrobiOLogy
 PaleontOLogy
PaleontOLogy
 ZoOLogy
ZoOLogy
 AnthropOLogy
AnthropOLogy
 ArchaeOLogy
ArchaeOLogy
 Astronomy
Astronomy
 Climate Change
Climate Change
 Earth
Earth
 Physics
Physics
 Water
Water
