
To keep track of the elements, scientists use the Periodic Table, a chart that shows all the elements. (Click here for a pdf version of the Periodic Table.) Scientists can quickly find out basic information about an element just by looking at the Periodic Table.
On the periodic table, elements are listed in order of increasing atomic number.
Elements in the same row are in the same period. This means they have similar physical properties, such as how well they bend or conduct electricity.
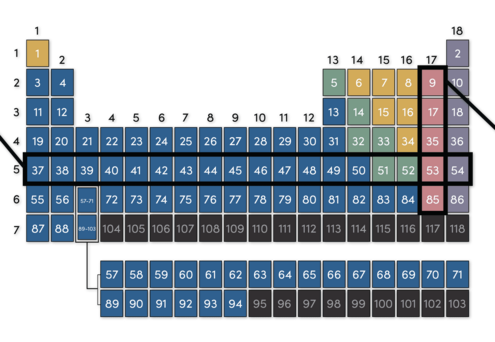
Elements in the same column are in the same group. This means they react with other elements in similar ways.
Here's a close-up look at the carbon square from the Periodic Table.
Elements in the same row are in the same period. This means they have similar physical properties, such as how well they bend or conduct electricity.
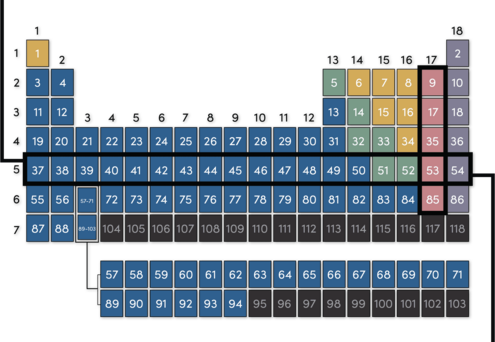
Elements in the same column are in the same group. This means they react with other elements in similar ways.
Here's a close-up look at the carbon square from the Periodic Table.
Atomic Number: the number of protons in the nucleus (which is the same as the number of electrons in the atom).
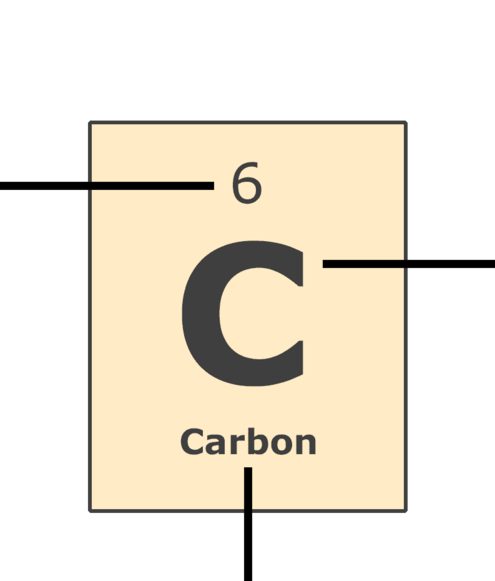
Symbol: a one or two letter symbol that represents the element. These internationally-used symbols are abbreviations for the common name or the Latin name of the element.
Name: the element's common name.
Atomic Number: the number of protons in the nucleus (which is the same as the number of electrons in the atom).
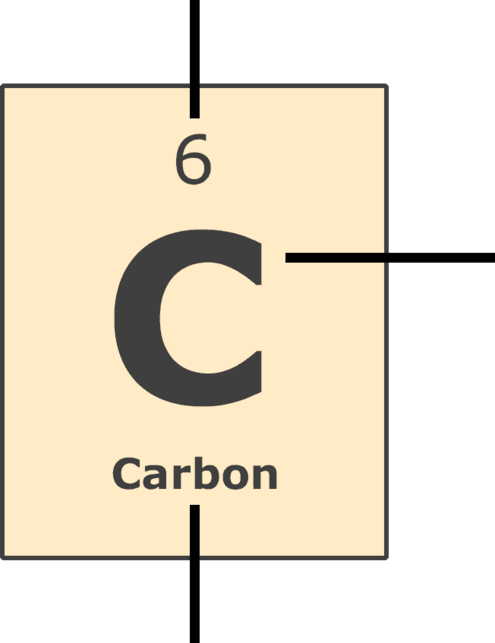
Symbol: a one or two letter symbol that represents the element. These internationally-used symbols are abbreviations for the common name or the Latin name of the element.
Name: the element's common name.
Atomic Number: the number of protons in the nucleus (which is the same as the number of electrons in the atom).
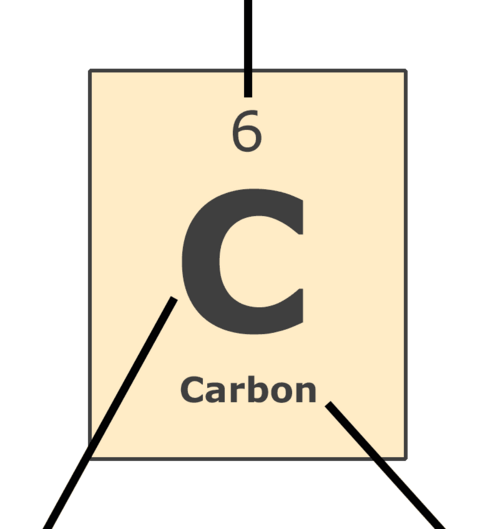
Name: the element's common name.
Symbol: a one or two letter symbol that represents the element. These internationally-used symbols are abbreviations for the common name or the Latin name of the element.




 Biodiversity
Biodiversity
 Brain
Brain
 Genetics
Genetics
 Marine BiOLogy
Marine BiOLogy
 MicrobiOLogy
MicrobiOLogy
 PaleontOLogy
PaleontOLogy
 ZoOLogy
ZoOLogy
 AnthropOLogy
AnthropOLogy
 ArchaeOLogy
ArchaeOLogy
 Astronomy
Astronomy
 Climate Change
Climate Change
 Earth
Earth
 Physics
Physics
 Water
Water
